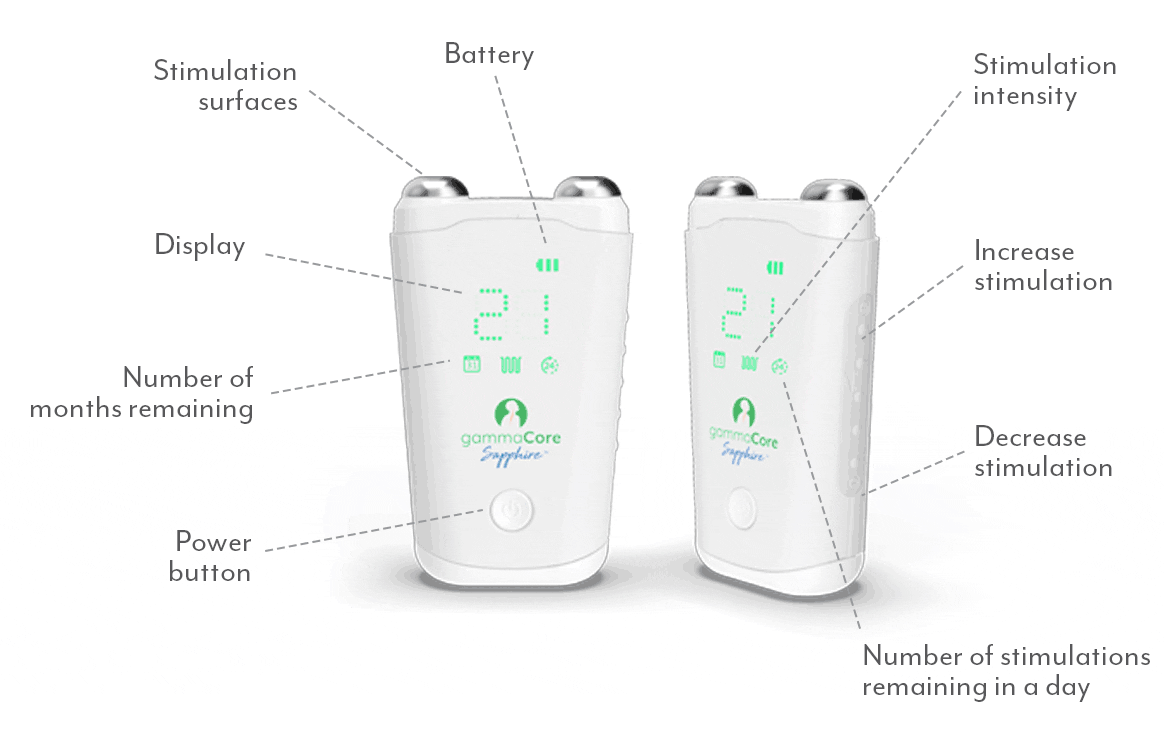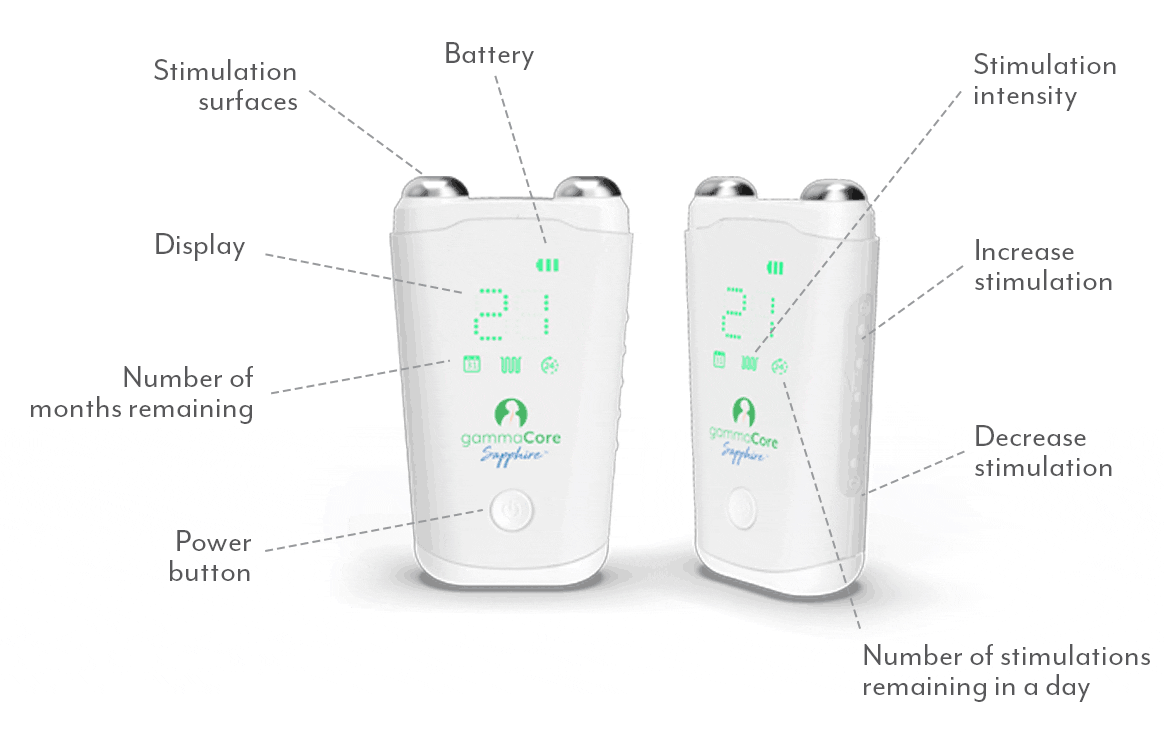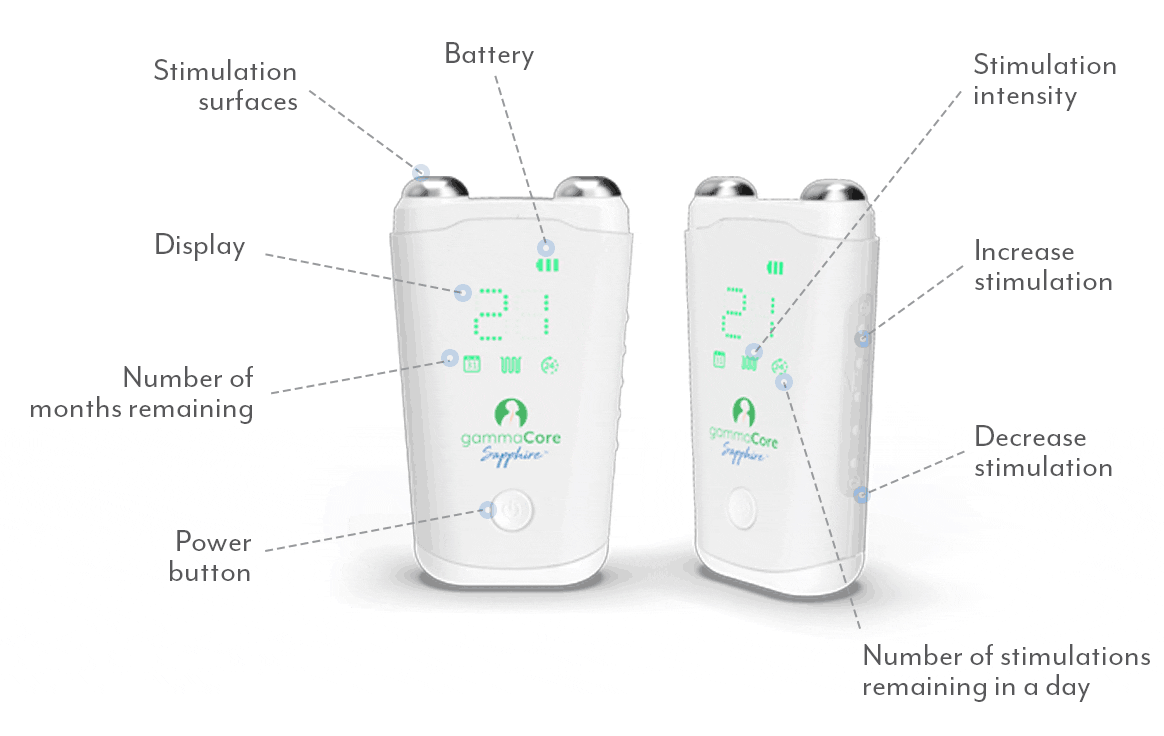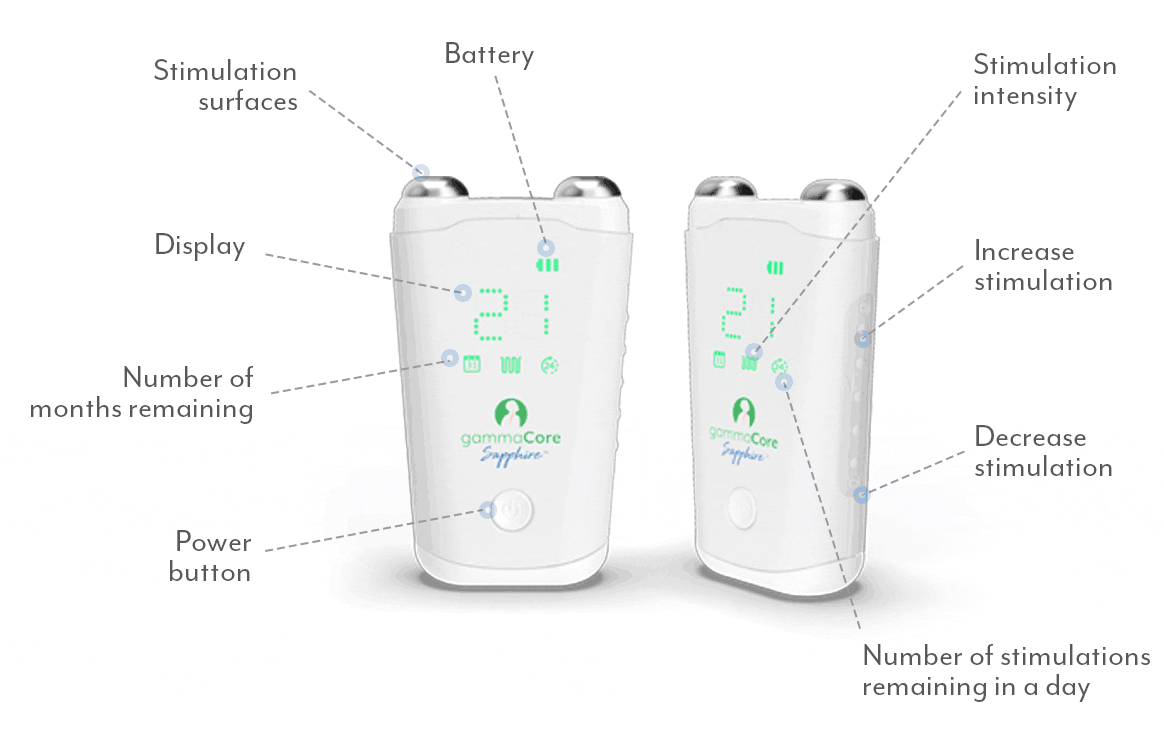
Frequently Asked Questions:
What is gammaCore?
gammaCore™ non-invasive vagus nerve stimulator (nVNS) is a small, handheld, non-invasive vagus nerve stimulator with 6 FDA-cleared indications for headache, including migraine and cluster headache. It is an easy-to-use, drug-free medical device used to help prevent headache pain before it starts or quickly stop painful attacks when they happen. gammaCore nVNS is available by prescription from your health care provider.
For full Indications and Important Safety Information, visit https://gammacore.com/about/important-safety-information/.
Is gammaCore FDA-cleared?
Yes, gammaCore has 6 FDA-cleared indications for headache. Please see below for more details:
gammaCore™ (non-invasive vagus nerve stimulator) is intended to provide non-invasive vagus nerve stimulation (nVNS) on the side of the neck. gammaCore is indicated for:
- The preventive treatment of migraine headache in adolescent (age 12 and older) and adult patients.
- The acute treatment of pain associated with migraine headache in adolescent (age 12 and older) and adult patients.
- Adjunctive use for the preventive treatment of cluster headache in adult patients.
- The acute treatment of pain associated with episodic cluster headache in adult patients.
- Treatment of hemicrania continua and paroxysmal hemicrania in adults.
- The effectiveness of gammaCore has not been established in the acute treatment of chronic cluster headache.
- gammaCore is contraindicated for patients if they:
- Have an active implantable medical device, such as a pacemaker, hearing aid implant, or any implanted electronic device
- Are using another device at the same time (e.g., TENS Unit, muscle stimulator) or any portable electronic device
- Safety and efficacy of gammaCore have not been evaluated in the following patients:
- Patients diagnosed with narrowing of the arteries (carotid atherosclerosis)
- Patients with a metallic device, such as a stent, bone plate, or bone screw, implanted at or near the neck
- Patients who have had surgery to cut the vagus nerve in the neck (cervical vagotomy)
- Pediatric patients (younger than 12 years
- Pregnant women
- Patients with clinically significant hypertension, hypotension, bradycardia, or tachycardia
NOTE: This list is not all inclusive. Please refer to the gammaCore Instructions for Use for all of the important warnings and precautions before using or prescribing this product.
What does a gammaCore treatment feel like?
When using gammaCore, you should feel a tingling sensation on the neck where the device is held, spreading towards the corner of your mouth. You might also notice a slight downward pulling on your lip, which is a common muscle contraction indicating correct treatment positioning. Users can adjust the intensity level at any time to ensure comfort. The treatment should never be painful.
For more information on using gammaCore, visit https://www.gammacore.com/how-to-use-gammacore/
How long are treatments?
When using gammaCore for preventive purposes, treatments typically last 4 minutes, consisting of two 2-minute stimulations on the same side of the neck, twice daily (morning and night)*. For acute attacks, follow a protocol of two 2-minute stimulations on the same side of the neck as needed, with repeated treatment if pain persists. Learn more about treatment timing at https://gammacore.com/getting-started/dosing/.
*Additional treatments may be recommended for cluster headache and other trigeminal autonomic cephalalgias.
What intensity level do I use?
For best results, increase the intensity until you feel a tingling sensation on the neck that spreads towards the corner of your mouth, and a slight "lip pull" sensation. Most users find this occurs between 15 and 25 on the 0-40 intensity scale. You may need to adjust the position or angle of the device to achieve this sensation. The optimal therapeutic intensity level varies for each individual.
For more information on using gammaCore, visit https://www.gammacore.com/how-to-use-gammacore/.
Can I use gammaCore with other medications?
Yes, gammaCore can be used alongside other medications. It is designed to be a complementary therapy. However, it's always important to discuss any new treatments with your health care provider to ensure there are no specific contraindications or interactions based on your individual health needs and current medications. Your health care provider can provide personalized advice and adjust your treatment plan as necessary.
Talk to your health care provider to determine if gammaCore is right for you. For full Indications and Important Safety Information, visit https://gammacore.com/about/important-safety-information/.
How long does the device last?
The gammaCore device offers multiple therapy options prescribed by a licensed health care provider. Available durations are 3, 12, and 36 months of consecutive treatment. Consult your health care provider to determine the appropriate prescription length for your needs. Please refer to the Important Safety Information and Instructions for Use for further details.
Are there any side effects when using gammaCore?
gammaCore is a safe and well-tolerated non-drug treatment. Most reported side effects are mild, occur during device use, and disappear soon after each treatment. The most common side effects, occurring in less than 2% of patients, include the following:
- Application site discomfort, irritation, and redness
- Muscle twitching and/or contractions
- Local pain in the face, head, or neck area (including toothache)
- Headache
- Dizziness
- Tingling, pricking, or a “pins and needles” sensation at the application site
For full Indications and Important Safety Information, visit https://gammacore.com/about/important-safety-information/.
Can I travel with gammaCore?
Traveling with gammaCore is safe. The device contains a lithium battery, so it's best to store it in your carry-on luggage to avoid extreme conditions. Additionally, one conductive gel tube complies with TSA liquid carry-on regulations.
What is the difference between gammaCore and other treatment options?
See the comparison chart below for some differences between gammaCore and other treatments.
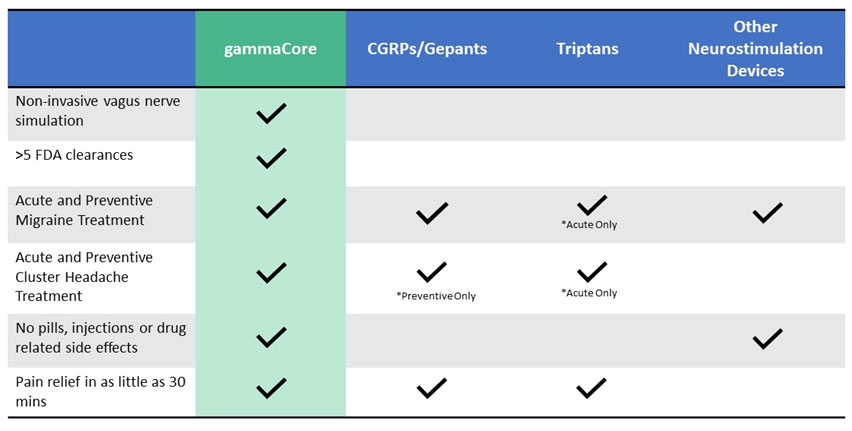
*Always speak with your health care provider to determine the best option for you.
How does gammaCore work?
gammaCore works by sending gentle electrical stimulation to the vagus nerve, which helps manage pain signals in the brain. During treatments, gammaCore is placed on either side of the neck where the vagus nerve is located. The device sends patented electrical pulses through the skin to stimulate the vagus nerve. This stimulation prompts the vagus nerve to send messages to the brain, alleviating symptoms and providing fast relief. This action can also help prevent future migraine and cluster headache attacks.
What conditions can gammaCore treat?
gammaCore is FDA-cleared with 6 indications for headache. It is intended for preventive and acute migraine treatment in adolescents and adults ages 12 and up, as well as adult acute and preventive treatment of cluster headache, and the treatment of hemicrania continua and paroxysmal hemicrania.
For full Indications and Important Safety Information, visit https://gammacore.com/about/important-safety-information/.
Is gammaCore safe to use?
Yes, gammaCore is FDA-cleared with robust scientific and clinical data in migraine to support its safe and effective use, including:
- 7 randomized controlled clinical trials
- >30 mechanism of action peer-reviewed papers, and
- >40 peer-reviewed clinical papers.
For more information on our clinical studies, visit: https://gammacore.com/prescribing-gammacore/clinical-efficacy/
Talk to your health care provider to determine if gammaCore is right for you. See Important Safety Information and Instructions for Use.
How do I get gammaCore?
gammaCore is available by prescription only from your health care provider. If you have a prescription, fax it to
855-647-1320 or email orders@electrocore.com. If you need a prescription, download the prescription form and have your health care provider send it over.
Need a health care provider? Search our clinic finder to find a provider near you at
https://www.gammacore.com/clinic-finder/.
Active-duty military and Veterans can obtain a prescription through the VA. Learn more about the process at https://www.gammacore.com/for-veterans/.
Is gammaCore covered by HSA/FSA?
Yes, your gammaCore expenses may be eligible for reimbursement through your Health Savings Account (HSA) or Flexible Spending Account (FSA). Patients can utilize their HSA or FSA funds to purchase gammaCore, offering a potentially tax-advantaged way to manage treatment costs. Please consult your specific HSA/FSA plan provider to confirm coverage and any required documentation.
How quickly can I expect relief with gammaCore?
gammaCore therapy results vary among individuals. Some patients may experience rapid relief after an acute treatment at the onset of migraine or episodic cluster headache pain. Using the device several times a day as a preventive therapy may reduce the severity, duration, and frequency of certain headaches and potentially decrease reliance on headache medications. Your doctor will determine the best nVNS treatment regimen for you. Most patients see a response within 3-5 acute attacks or after 4-8 weeks of preventive therapy. An adequate trial is essential to assess your response to gammaCore therapy.
What is the vagus nerve?
The vagus nerve is the longest nerve that connects directly to the brain. It branches to the heart, lungs, stomach, and other organs. It acts as the nervous system's "superhighway," facilitating communication between the brain and various body parts. This nerve primarily functions as a sensory nerve, reporting information to the brain and transmitting instructions to the body. It influences many systems, including pain regulation, anxiety, fatigue, heart rate, digestive activity, and lung function.
What is vagus nerve stimulation?
Vagus nerve stimulation (VNS) involves using electrical impulses to activate (or stimulate) the vagus nerve, which runs from the brainstem through the neck and then to the abdomen. This therapy is used to influence various bodily functions, such as heart rate, digestion, and the autonomic nervous system, and it has applications in treating conditions like migraines. By activating the vagus nerve, VNS helps regulate pain signals and other neural pathways, providing relief from specific symptoms. For more information on VNS and its benefits, consult a health care provider.
How does gammaCore stimulate the vagus nerve?
gammaCore delivers gentle electrical stimulation to the vagus nerve through the skin. During treatments, the device is placed on either side of the neck where you find your pulse, and where the vagus nerve is located. The patented electrical energy from gammaCore stimulates the nerve, prompting it to send messages to the brain. This stimulation works to alter pain signals, offering fast relief and preventing future migraine or cluster headache attacks.
Is gammaCore a TENS Unit?
No, gammaCore is not a TENS (Transcutaneous Electrical Nerve Stimulation) unit. While both devices use electrical stimulation, they serve different purposes and function differently. gammaCore is a non-invasive vagus nerve stimulator (nVNS) specifically designed to stimulate the vagus nerve through the skin at the neck, using a proprietary signal to alter pain signals and other neurological pathways. In contrast, TENS units are generally used for peripheral nerve stimulation to relieve localized pain and are not designed to target the vagus nerve. Additionally, TENS units are unsafe to use on the neck, while gammaCore is specifically designed for safe application. For more information, visit Why TENS Units Fall Short for Vagus Nerve Stimulation.
How often should I use gammaCore?
For preventive treatment, use gammaCore twice daily. This involves two 2-minute stimulations on the same side of the neck, both in the morning and at night. For attacks, use gammaCore when symptoms arise, performing two 2-minute stimulations on the same side of the neck, and repeating if the pain persists.
Please note that additional treatments may be recommended for cluster headache and other trigeminal autonomic cephalalgias. Consult your health care provider for personalized recommendations.
How do I use gammaCore?
Follow these 3 simple steps to optimize each treatment session:
1. Use 2 fingers to locate your pulse on either side of your neck. The vagus nerve is located in the same area.
2. Remove the cap. Apply a pea-sized amount of the provided gel to each stimulation surface (other gels will not work). Turn on gammaCore.
3. Place gammaCore over the treatment location. Increase the stimulation intensity until you notice slight muscle contractions at the corner of your mouth. Many users stay between 15-25.
You can also watch our How to Use gammaCore video below for more instructions.
Why do I need gel to use gammaCore?
The conductive gel is essential for effective gammaCore use because it facilitates the transmission of electrical signals from the device to the vagus nerve. Applying the gel to the treatment area on your neck ensures proper contact and reduces resistance, allowing the device to deliver consistent and effective therapy. Always apply the provided gel (other gels will not work) as instructed to optimize your treatment experience.
Watch the video below to learn how to properly apply gel when using the gammaCore device.
Do I need a prescription for gammaCore?
Yes, gammaCore is a prescription-based medical device. You will need a prescription from a licensed health care provider to use it.
If you think gammaCore might be right for you, please consult with your health care provider. To find a provider near you, use our clinic finder tool to locate a health care professional who can help you determine if gammaCore would be a suitable treatment for your migraine and/or headache pain.
For full Indications and Important Safety Information, visit https://gammacore.com/about/important-safety-information/.
How long should I use gammaCore?
Results with gammaCore vary among individuals. Some patients experience lasting improvement after a defined period of use, while others may see symptoms return upon discontinuation and choose to continue therapy. Discuss the ongoing use of gammaCore with your health care provider.